
Dirhenium heptaoxide
Other name: Rhenium(VII) oxide; trioxo-(trioxorheniooxy)rhenium; trioxo(trioxorheniooxy)rhenium
CAS no. : 1314-68-7
EINECS no. : 215-241-9
Molecular formula: O7Re2
Molecular weight: 484.4098
Density: 6.103 g/mLat 25 ° C (lit.)
Melting point: 220 ° C (lit.)
Boiling point: 360 ° C (lit.)
Flash: 360 ° C
- 描述
- Inquiry
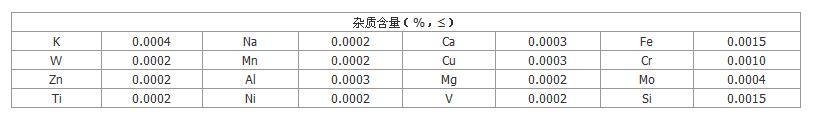
Chemical Specification: Re2O7≥99.99%, Re≥76.87%,
impurities<100ppm
Appearance and character: yellow to green powder
Specific heat capacity (solid state) : 0.3429 J/(g K)
Molar heat capacity (solid) : 166.1 J/ mol K
Specific bonding free energy (gaseous) : -2.052 kJ/g; (solid state) : -2.201 kJ/g
Molar bonding energy (gaseous state) : -994 kJ/mol; (solid state) : -1066 kJ/mol
Specific bonding thermal energy (gaseous) : -2.271 kJ/g|; (solid state) : -2.56 kJ/g
Molar bonding heat (gaseous) : -1100 kJ/mol; (solid state) : -1240 kJ/mol
Molar diffusion heat: 65.7 kJ/mol
Specific heat of diffusion: 0.136kj /g
Molecular structure
X – ray single crystal diffraction confirmed that in dirhenium septicum crystals, one rhenium atom was coordinated with oxygen atom by a twisted octahedron and one rhenium atom by a tetrahedron. The octahedron shares a vertex with the tetrahedral structure, and the octahedron is also connected with another octahedral common side. In the gaseous state, rhenium septicum exists as a molecule with two ReO4 tetrahedral structures sharing one vertex.
The experiment for making
Metal rhenium in more than 150 ° C in air or oxygen combustion, strong or rhenium dioxide in air heat can generate seven oxide rhenium, low oxygen concentration can also be observed when red rhenium oxide generated. In addition, the rhenic acid solution was prepared by metal rhenium acting with hydrogen peroxide, and then co-heating with phosphorus pentoxide under vacuum.
Rhenium septicum can be purified by vacuum sublimation to make large yellow angular crystal
A chemical reaction
Sevoxide dirhenic dissolved in water to produce rhenic acid solution:
Re2O7 + H2O = 2HReO4
Reduction by hydrogen gas can be used to obtain the peroxide rhenium and metal rhenium successively.
The synthesis of methyl rhenium trioxide (“MTO”) is a new type of highly efficient catalyst
Purpose: catalyst. Oxidant. alloy
Industrial production method
Oxidation of metal rhenium. The metal rhenium powder heated to above 150 ℃ in the oxygen flow is generated seven oxide rhenium. However, the oxidation reaction has to go through different stages, so the preparation of pure rhenium septicum should be carried out under the specified reaction conditions in the closed system. 2g metal rhenium was installed at the closed end of the Pyrex glass reaction tube. The reaction tube has a volume of about 1L and is placed across. Dry the tube thoroughly. The oxygen was sent to the reaction tube through the three-way cock, and the internal pressure was maintained at 101.325kPa. Mobile heating to 400 ~ 425 ℃ in advance of the short tube furnace, starting from the closed end of the reaction tube heating, heating lasts about 2 h after, will move to a move from the tubular furnace heating. When heated for about 6h, bright yellow rhenium oxide is produced. As the compound is condensed in the cold area after sublimation, the mobile tube furnace can control its precipitation and collect it, and thus can be purified. In addition, the compound is hygroscopic, so the reaction tube should be sealed and preserved.
相关产品
-
Digallium triselenide powder
English alias: Gallium (III) selenide; gallium selenide (2: 3)
CAS number: 12024-24-7
EINECS No. 234-693-8
Molecular formula: Ga2Se3
Molecular weight: 376.326
-
Gallium trichloride
English alias:; Gallium (III) chloride ,Gallium chloride anhydrous; Gallium chloride
CAS number: 13450-90-3
EINECS number: 236-610-0
Molecular formula: Cl3Ga
Molecular weight: 176.082
Steam pressure: 33900 mmHg at 25 ° C
-
Germanium telluride
Other name: Germanium(II) telluride
CAS no. : 12025-39-7
EINECS no. : 234-706-7
Molecular formula: GeTe
Molecular weight: 200.24
Density (g/mL,25℃) : 6.14
Melting point (oC) : 725 -
Tellurium tetrachloride
Other name: Tellurium(IV) chloride, tetrachloro-lambda~4~-tellane
CAS no. : 10026-07-0
EINECS no. : 233-055-6
Molecular formula: H6Cl4Te.
Molecular weight: 275.4596
Melting point : 224 ° c.
The boiling point: 380 ° c.
-
Indium telluride
Other name: indium(iii) telluride
CAS no. : 1312-45-4
EINECS no. : 215-194-4
Molecular formula: In2Te3
Molecular weight: 612.44
Density (g/mL,25℃) : 5.78
Melting point (oC) : 667 -
Ammonium perrhenate
Purity: 4N, 5N
4N: NH4ReO4≥99.99% , Re ≥69.4%
Appearance : white flake crystal.
Application: rhenium compound standard sample; Rhenium addition; Production of rhenium metal products, rhenium alloy and rhenium compounds raw materials. -
Diindium triselenide
Other name: indium selenide ,Indium(III)selenide, indium selenide (2:3)
CAS no. : 12056-07-4
EINECS no. : 235-016-9
Formula: In2Se3
Molecular weight: 466.516
Density :5.80 g/cm³
-
Rhenium trichloride
Rhenium trichloride ReCl3 Re≥35% Purity: 99%
Properties: Dark red or purple crystals. Melting point 727℃. Boiling point 800 ~ 850℃. 500℃ vacuum sublimation, vapor green. Soluble in water, acid, base, liquid ammonia and ethanol, slightly soluble in ether. -
Rhenium dioxide ReO2
Specification: ReO2≥99.99%, Re≥85.33%
Impurity:<100ppm
Appearance: black gray particulate matter. -
Digallium trioxide
Other name: GALLIUM SESQUIOXIDE;GALLIUM OXIDE;GALLIUM(III) OXIDE;GALLIUM(+3)OXIDE;digalliumtrioxide;Ga2-O3;Gallia
CAS number: 12024-21-4
EINECS No. 234-691-7
Molecular formula: Ga2O3













