
Lanthanum Strontium Manganite
Lanthanum Strontium Manganite powder
Manganese acid strontium lanthanum
Molecular formula: La1-xSrxMnO3-d
Usage: Solid fuel battery target material, catalyst target materials, superconducting substrate, the semiconductor material.
Origin: China
- 描述
- Inquiry
******* Related Information*******
Doping Modification and Double Negative Mechanism of Lanthanum Manganate
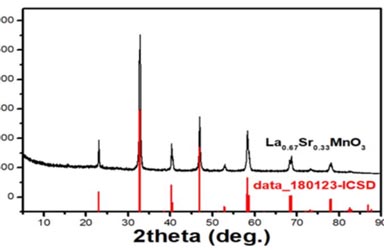
In recent years, negative material properties such as negative dielectric constant, negative magnetic permeability, negative refractive index, negative Poisson’s ratio, negative mass density and other negative material properties, as metamaterials (metamaterials, also known as metamaterials) Learning, microwave, optics, acoustics, mechanics, thermal studies and other aspects of the deepening and increasingly prominent scientific significance and application value, more and more attention. However, with the negative dielectric constant, negative permeability as the main performance characteristics of the “super” material, since 2000 was put forward, does not emphasize the chemical composition, micro-organization, and “real” material far. Subsequently, the material workers began to explore the conventional double-negative materials based on intrinsic properties using typical material techniques and found double negative properties in metal / ceramic hetero composites. (La1-xSrxMnO3, LSMO) contains a wealth of physical phenomena such as double exchange, electro-phonon coupling, hyper-exchange, and coulomb, and leads to charge, spin, orbital order, magnetic field or light induced Insulator – metal transition, phase separation and giant magnetoresistance effect. In addition, the single electron band of the electrons of LSMO is wider than that of lanthanum manganate (La1-xCaxMnO3, LCMO), the material has a ferromagnetism in a wide doping concentration range, and the Curie temperature is higher (Tc = 350 -370K), so it has more advantages and practical value. However, there are few reports on the spectral characteristics such as dielectric constant and magnetic permeability of LSMO. Therefore, this kind of single-phase material based on the intrinsic nature of its dual negative research is a very meaningful work. In this paper, LSMO samples with different strontium content and multi-element doping were prepared by sol-gel self-propagating combustion method. The microstructures of the samples were analyzed by X-ray diffraction (XRD) and scanning electron microscopy (SEM) The effects of composition, crystal structure and microstructure on the electromagnetic parameters of LSMO ceramics were studied systematically by means of impedance analyzer, frequency response / characteristic analyzer, four-probe resistivity / resistance tester and so on. The relationship between the intrinsic dielectric constant and the chemical composition and the permittivity and permeability of the intrinsic permittivity were revealed. The specific research contents are as follows: (1) LS1-xSrxMnO3 ceramics with different Sr contents were prepared by sol-gel self-propagating combustion method under different sintering temperature and holding time. The sintering temperature and And the influence of the parameters such as the holding conditions on the phase composition and microstructure of the composites were investigated. The effects of the phase composition and microstructure on the dielectric properties, electrical impedance, electrical conductivity and permeability were also analyzed. With the increase of sintering temperature, the heterogeneous phase gradually disappears, and the grain size increases and tends to be uniform. On this basis, the sintering temperature and holding time were optimized. (2) The effects of different Sr contents on microstructure and electromagnetic properties were studied. It was found that with the increase of Sr content, the crystal symmetry was improved, and the ferromagnetism of La1-xSrxMnO3 was improved. In addition, due to the different content of Sr, resulting in three different dielectric polarization mechanism, in addition to Debye relaxation, Lorentze resonance and Drude plasma oscillation of these two mechanisms can produce negative dielectric constant (ε’0). The negative permeability (μr’0) for La1-xSrxMnO3 can be achieved by adjusting the Sr content in different frequency bands to achieve the double negative properties of the material in a specific frequency band. (3) It is studied that the doping of La0.7Sr0.3MnO3, Fe, Ni, Cu and Co, which is doped with B-element, leads to the change of lattice distortion and grain morphology, which improves the symmetry of crystal and thus becomes effective The regulation of dielectric properties and conductivity. For tuned ceramic applications, this tunability is very meaningful. Among them, the Ni element doped La0.7Sr0.3Mn0.5Ni0.5O3 has higher dielectric loss and better electromagnetic matching properties (relatively low □ r ‘), is conducive to absorbing. In addition, the experimental results of dielectric constant were examined by K-K relationship. (4) The dielectric dispersion characteristics of La1-xSrxMnO3 were calculated by Lorentz and Drude models. The results show that the negative permittivity is caused by Lorentz resonance when the Sr content is low (eg x = 0.1). When the Sr content is high (such as x≥0.2), the increase of the conduction electron concentration Under the action of the alternating electric field, a collective plasma oscillation occurs, resulting in a plasma oscillation type negative dielectric. The existence of these two negative dielectric mechanisms is further demonstrated by the analysis of the conductivity. (5) The impedance of La1-xSrxMnO3 was analyzed by equivalent circuit method and its equivalent circuit was obtained. The electrical performance at high frequency was studied. When the sintering temperature and Sr content are low, the sample can be equivalent to the circuit composed of capacitance and resistance. When the sintering temperature and Sr content are high, the sample can be equivalent to the circuit composed of capacitance, resistance and inductance. And found that the generation of parallel inductors may be the source of negative permittivity. (6) The permeability relationship of La1-xSrxMnO3 with frequency is fitted by the permeability formula. The results show that the generation of negative magnetic permeability is the result of domain wall resonance and natural resonance, and the magnetic anisotropy and lattice constant are adjusted by Sr element doping, so that the resonant frequency of domain wall moves to the low frequency direction, And then to achieve the negative magnetic permeability band regulation. By adding a constant magnetic field (Hdc = 0.55 T), the resonance intensity of negative magnetic permeability can be enhanced, and the negative permeability values and frequency bands can be regulated. The work of this thesis is supported by scientific research projects such as the National Natural Science Foundation of China and the New Century Talent Support Program of the Ministry of Education.
相关产品
-
Europium Nitride
Molecular Formula: EuN
Molecular Weight: 165.97
CAS No.: 12020-58-5
EINECS No.: 234-659-2
-
Thuliium acetylacetonate trihydrate
Other name: THULIUM 2,4-PENTANEDIONATE, TRIHYDRATE, THULIUM (III) ACETYLACETONATE TRIHYDRATE; Thuliiumacetylacetonate
CAS no. : 14589-44-7
Molecular formula: C15H21O6Tm
Molecular weight: 466.2578
Melting point: 121-123℃
-
Lutetium fluoride
Other name: Lutetium Fluoride Anhydrous,Lutetium trifluoride,Lutetium(III) fluoride
Chemical formula: LuF3
CAS: 13760-81-1
EINECS: 237-355-8
Melting point: 1182 ℃
Boiling point: 2200 ℃
Density: 8.33 g/mlDescription: white powder, no crystal water. Insoluble in water, hydrochloric acid, nitric acid and sulfuric acid, but soluble in perchloric acid.
-
Neodymium metaphosphate
Laser glass additive Neodymium phosphate
English name: Neodymium metaphosphate
Chemical formula: Nd (PO3) 3
-
Nano Dysprosium Oxide
Other name: Didysprosium trioxide
CAS no. : 1308-87-8
EINECS no. : 215-164-0
Molecular formula: Dy2O3
Molecular weight: 372.9982
Melting point: 2330-2350 ℃
Solubility: insoluble
-
Lanthanum acetylacetonate hydrate
Other name: LaAA , Lanthanum(III) Acetylacetonate hydrate, Tris(2,4-pentanedionato)lanthanum(III); Lanthanum (III) 2,4-Pentanedionate; 2,4-Pentanedione Lanthanum Derivative Hydrate; Tris(Acetylacetonato)Lanthanum N-Hydrate; Lanthanum Tri(Acetylacetonate)
CAS no. : 64424-12-0
EINECS no. : 238-187-8
Molecular formula: C5H9LaO3.
Molecular weight: 256.0275.
-
Praseodymium fluoride
Other name: Praseodymium trifluoride
CAS no. : 13709-46-1
EINECS no. : 237-254-9
Formula: F3Pr.
Molecular weight: 197.9029
-
Lanthanum manganate
Purity: not less than 99.9%
Application: Solid fuel cells, semiconductors, catalysts
-
Lanthanum titanate
Other name: Lanthanum titanium oxide, Lanthanum (+ 3) cation;
Oxygen anion (2); Titanium (+ 4) cation; Lanthanum (3 +); Oxygen (2 -) dihydride; titanium
CAS no. : 12031-47-9; 144087-28-5
EINECS no. : 234-751-2
Formula: La2O7Ti2
Molecular weight: 485.54 -
Thulium fluoride
Other name: Thulium Fluoride Anhydrous,Thulium trifluoride,Thulium(III) fluoride
CAS no. : 13760-79-7
EINECS no. : 237-353-7
Molecular formula: TmF3
Molecular weight: 187.9315
Melting point: 1158 ℃
Boiling point: 2227 ℃
Density: 7.9 g/ml













