
Rhenium trichloride
Rhenium trichloride ReCl3 Re≥35% Purity: 99%
Properties: Dark red or purple crystals. Melting point 727℃. Boiling point 800 ~ 850℃. 500℃ vacuum sublimation, vapor green. Soluble in water, acid, base, liquid ammonia and ethanol, slightly soluble in ether.
- 描述
- Inquiry
Other name: Rhenium chloride
Molecular formula: ReCl3
Genus: Reduced to metal ruthenium at 250-300 °C
The aqueous solution was red and could not precipitate silver chloride from silver nitrate solution. It can be extracted by ether. The hydrolytic rhenium Re2O3·xH2O is slowly hydrolyzed, but it is very stable in the aqueous solution of hydrochloric acid. It is not oxidized by potassium permanganate or chlorine water at room temperature. The polymer (ReCl3)3 obtained by sublimation is a cluster compound. Under the condition of 250 ~ 300 ℃ by hydrogen reduction of metal rhenium. Rhenium tetrachloride and rhenium monochloride were formed by heating in oxygen. It was prepared by pyrolysis of pentachloride rhenium in nitrogen. It is an important raw material for preparing rhenium inorganic and organic compounds.
相关产品
-
Tellurium tetrachloride
Other name: Tellurium(IV) chloride, tetrachloro-lambda~4~-tellane
CAS no. : 10026-07-0
EINECS no. : 233-055-6
Molecular formula: H6Cl4Te.
Molecular weight: 275.4596
Melting point : 224 ° c.
The boiling point: 380 ° c.
-
Lanthanum strontium gallium magnesium oxide
Lanthanum strontium gallium magnesium oxide (LSGM) is a ceramic electrolyte material with high ionic conductivity. LSGM is used as an electrolyte membrane to improve SOFC performance or reduce operating temperature. The ionic conductivity of LSGM is about twice that of yttrium-stabilized zirconia (YSZ-8). This LSGM powder is ideal for use in casting, ink formulation, pelletizing and other ceramic manufacturing processes.
-
Digallium trioxide
Other name: GALLIUM SESQUIOXIDE;GALLIUM OXIDE;GALLIUM(III) OXIDE;GALLIUM(+3)OXIDE;digalliumtrioxide;Ga2-O3;Gallia
CAS number: 12024-21-4
EINECS No. 234-691-7
Molecular formula: Ga2O3
-
Gallium trichloride
English alias:; Gallium (III) chloride ,Gallium chloride anhydrous; Gallium chloride
CAS number: 13450-90-3
EINECS number: 236-610-0
Molecular formula: Cl3Ga
Molecular weight: 176.082
Steam pressure: 33900 mmHg at 25 ° C
-
Digallium triselenide powder
English alias: Gallium (III) selenide; gallium selenide (2: 3)
CAS number: 12024-24-7
EINECS No. 234-693-8
Molecular formula: Ga2Se3
Molecular weight: 376.326
-
Diindium triselenide
Other name: indium selenide ,Indium(III)selenide, indium selenide (2:3)
CAS no. : 12056-07-4
EINECS no. : 235-016-9
Formula: In2Se3
Molecular weight: 466.516
Density :5.80 g/cm³
-
Gallium trichloride GaCl3
Purity: 99.99%
Physical characteristics: Strong hydrolysis in water, produce smoke in moist air. Melting point: 77.9℃ Boiling point: 201.03℃ Density: 2.47g/cm³
Appearance: White crystal or powder. -
Germanium telluride
Other name: Germanium(II) telluride
CAS no. : 12025-39-7
EINECS no. : 234-706-7
Molecular formula: GeTe
Molecular weight: 200.24
Density (g/mL,25℃) : 6.14
Melting point (oC) : 725 -
Dirhenium heptaoxide
Other name: Rhenium(VII) oxide; trioxo-(trioxorheniooxy)rhenium; trioxo(trioxorheniooxy)rhenium
CAS no. : 1314-68-7
EINECS no. : 215-241-9
Molecular formula: O7Re2
Molecular weight: 484.4098
Density: 6.103 g/mLat 25 ° C (lit.)
Melting point: 220 ° C (lit.)
Boiling point: 360 ° C (lit.)
Flash: 360 ° C
-
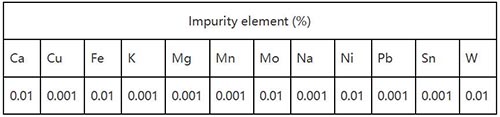
Rhenium dioxide ReO2
Specification: ReO2≥99.99%, Re≥85.33%
Impurity:<100ppm
Appearance: black gray particulate matter.













